John Samuelsen, Arkansas Archeological Survey
"Archeology is..." series - March 2025

A major focus of archeology is to understand what people did in the past. Archeological chemistry can answer questions about a large range of topics, but it is particularly useful for answering questions about the following:
-
Where people and animals are from
-
Using strontium and lead isotopes
-
-
Where artifacts are from
-
Using strontium and lead isotopes
-
Using instrument neutron activation analysis (INAA)
-
-
When something happened
-
Using radiocarbon dating (Carbon-14 dating) of plant material, bones, and teeth
-
-
What people ate or stored
-
Using carbon and nitrogen isotopes in bones and teeth
-
Using chromatography of residues in vessels
-
-
Climatic changes
-
Using oxygen isotopes
-
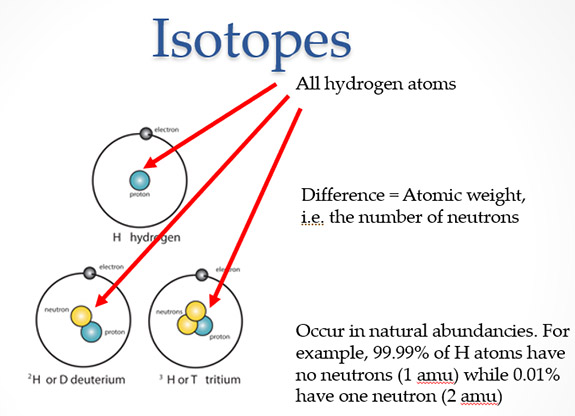
Here we will focus on two of the more common techniques utilized in archeology, the analysis of geologically sensitive elements (strontium and lead) and radiocarbon dating. All matter is made up of small particles called atoms, which have different properties depending on which element they are. Atoms of different elements are distinguished by the number of protons that make up their nucleus (those numbers on the periodic table of elements). Almost all nuclei also have neutrons. Many elements can have nuclei with more or fewer neutrons. This changes how much that particular atom weighs. These atoms of different weights, but with the same number of protons, are called isotopes. Chemists can use laboratory machinery to measure these small weight differences to see how many of each kind of isotope are present in a particular sample.
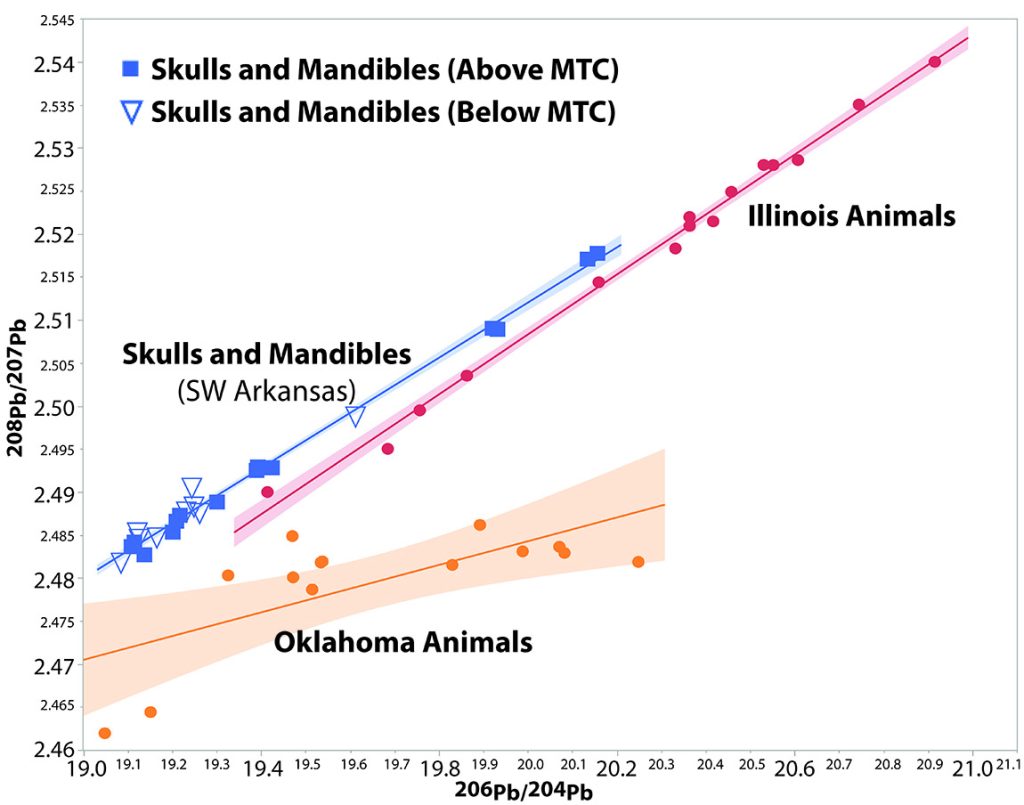
Strontium (Sr) and lead (Pb) originate in the earth’s crust with a known isotopic ratio. For Sr, the isotopic ratio of importance is 87Sr to 86Sr (how many atoms of 87Sr weight versus how many of 86Sr weight). For lead, 208Pb, 207Pb, 206Pb, and 204Pb are all important isotopes. As the rock in the earth’s crust forms and time passes, the ratio of these isotopes to each other will change based on the radioactive decay of other elements. Since different parts of the world have different amounts of these elements and are of varying ages, reflecting the local geology, it is possible to distinguish different geographic areas based on their isotopic signatures. For artifacts, this may enable determining the origin of an ore used in manufacturing a tool.
Humans and animals will absorb the Sr and Pb in their surrounding environment. This may be through eating food, drinking water, or breathing in pollution (these elements are always present in nature in small amounts). The same isotopic ratios that are present in the soil where the people grew up will be deposited in their bones and teeth. This forms an isotopic fingerprint that can identify the area where they grew up. In tooth enamel for example, this fingerprint does not change past childhood even if the person were to move to a new place and is locked into the enamel even after death (tooth enamel is formed in childhood and then does not change).